Publikationer pr. år
Publikationer pr. år
Blegdamsvej 3, 2200 København N.
Publikationer pr. år
Glycosylation is an essential cellular process that involves the covalent attachment of glycans (carbohydrates/sugars) to proteins; glycans play important roles in maintaining normal cellular functions and dysregulation of processes related to protein glycosylations are known to cause various diseases, e.g. cancer and developmental disorders. Our group is interested in the biosynthesis, regulation and biochemistry of O-linked glycosylations with a special focus on protein O-mannosylations (mannose-O-Ser/Thr). We use a combination of methods, including advanced mass spectrometry, to study structures, map site-specific locations and quantify changes of protein O-mannosylations on a proteome-wide scale. In addition, we use CRISPR/Cas9 gene editing for KO/KI of glycogenes in our efforts to study and understand enzymatic pathways involved in protein O-mannosylation.
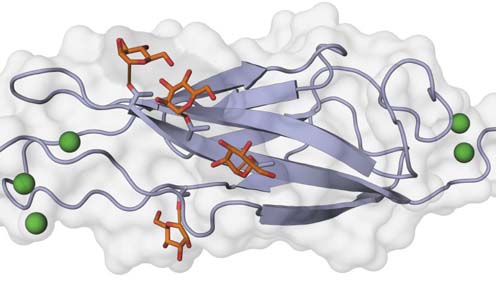
Cadherin O-mannosylation – the fourth extracellular cadherin (EC) domain of mouse E-cadherin is shown with O-linked mannose glycans (sticks) on specific β-strands. Calcium ions are depicted as green spheres. Illustration by Oliver Harrison, Julia Brasch and Lawrence Shapiro (Columbia University).
Cadherin-specific O-mannosylation by TMTC1-4
O-mannosylation was for a long time believed to be a rare type of protein modification in mammals. Until recently, α-dystroglycan (αDG), a component of the dystrophin complex, remained the only well-characterized protein with respect to O-mannosylation sites and structures. Building on the SimpleCell technology developed at Copenhagen Center for Glycomics (CCG), we established a glycoproteomic workflow for studying protein O-mannosylations on a proteome-wide scale. This approach allowed us to greatly expand the human O-mannose glycoproteome and led to the discovery of cadherins and protocadherins as major carriers of O-mannosylations. Using the SimpleCell technology, we recently uncovered that O-mannosylation of the cadherin superfamily is not mediated by the classical POMT1/POMT2 enzymes and identified the glycosyltransferase family (GT105), composed of TMTC1-4, that initiates the cadherin-specific O-mannosylation. Currently, we are characterizing the TMTC1-4 enzyme family, exploring the substrate specificities/interactomes of individual TMTC family members and studying the disease-causing mutations in this enzyme family. Our ambitions are to gain further understanding on cellular processes related to the function of cadherin-specific O-mannosylation in health and disease (e.g. Cobblestone lissencephaly).

Differential regulation of protein O-mannosylation in metazoans. O-mannosylation is predicted to be controlled by at least three distinct enzyme families; α-dystroglycan O-mannosylation is initiated by the POMT1/POMT2 family, the cadherin superfamily is glycosylated by the TMTC1-4 family and the plexin family (IPT/TIG domains) is O-mannosylated by unknown enzyme(s).
Identification of new genes and pathways involved in O-mannosylation and cell-cell communication
In addition to the cadherin superfamily, we have identified plexins as a major class of cell-surface O-mannosylated proteins. The IPT/TIG domains of plexins are frequently O-mannosylated on specific β-strands but, intriguingly, this O-mannosylation doesn’t seem to be mediated by POMT1/POMT2 or the TMTC1-4 family. Our results thus indicate that other, as yet unknown glycosyltransferases are present in mammalian systems and are responsible for directing O-mannosylation specifically to proteins with IPT/TIG folds. Using the SimpleCell platform, we are currently screening candidate genes to test this hypothesis and to identify the novel glycosyltransferase enzymes responsible for O-mannosylation in mammalian cells.
Nucleocytoplasmic O-mannosylation in yeast
All eukaryotes except yeast (e.g. S. cerevisiae) utilize a signaling mechanism that involves dynamic cycling of a sugar molecule on nucleocytoplasmic proteins, known as O-GlcNAcylation and dysregulation of this process is implicated in many common human illnesses e.g. diabetes, cardiovascular diseases and cancer. O-GlcNAcylation crosstalk with phosphorylation orchestrates essential cellular processes in all eukaryotic cells but the apparent lack of a nucleocytoplasmic O-GlcNAcylation system in yeast has been a longstanding conundrum. We recently discovered that yeast have a unique O-mannosylation capacity only found in nucleocytoplasmic compartments. The central hypothesis of this project suggests that yeast cells utilize the nucleocytoplasmic O-mannosylation machinery to modulate cellular processes, mirroring the signaling- and regulatory functions of the O-GlcNAcylation system of higher eukaryotes. Currently, we are exploring the dynamics of this nucleocytoplasmic O-mannosylation system and pursuing the hunt for the enzyme(s) responsible for nucleocytoplasmic O-mannosylation in yeast.

O-linked mannose glycosylation on nucleocytoplasmic and secreted proteins in yeast and metazoans. Phosphorylation and O-GlcNAcylation work together and orchestrate signaling processes in metazoans; yeast appears to have a similar system (based on O-linked mannose) operating in nucleocytoplasmic compartments; however, the functions and enzyme(s) in yeast remain unknown.
A. Halim, Oct, 2017
Curriculum Vitae for Adnan Halim
Personal information
ORCID: 0000-0003-1629-187X
Email: [email protected]
Education
2008-2012: PhD (Medical Science), Dept. of Clinical Chemistry and Transfusion Medicine, Sahlgrenska Academy, University of Gothenburg
2002-2007: MSc (Chemistry), University of Gothenburg
Academic appointments
2016: Visiting Associate Professor, Marie Curie fellow, Rockefeller University, New York, USA
2016: Associate Professor, Copenhagen Center for Glycomics, University of Copenhagen
2014-2016: Assistant Professor, Copenhagen Center for Glycomics, University of Copenhagen
2012-2014: Postdoctoral fellow, Copenhagen Center for Glycomics, University of Copenhagen
Supervisor and Teaching
2005-2017: Supervisor for 1 postdoc and 2 PhD students (1 finalized). Teaching experience from pre-graduate (Medicine, Pharmacy and Chemistry) and post-graduate programs (PhD courses).
Grants
2016: H2020-Marie Skłodowska-Curie Individual Fellowship
2016: The Novo Nordisk Foundation
Awards
2013: Honorary Phabian Award, Swedish Pharmaceutical Society
2012: Best thesis award, Institute of Biomedicine, University of Gothenburg
Bibliometric summary/citation information
Publications: 33 original papers and 3 reviews. (1x Cell, 1x Nat. Biotechnology, 1x Nat. Methods, 5x PNAS)
Impact: H-index: 18; total citations ~1100
Patent applications and issued patents
US patent: 8916387 B2, Diagnosis and treatment of Alzheimer's disease
US provisional application: N-glycosylation (WO2016091268 A2)
US provisional application: Production of N-glycoproteins for enzyme assisted glycomodification (WO2017008982 A1)
US provisional application: Yeast O-Mannose Nucleocytoplasmic glycosylation (US20170218032 A1)
I 2015 blev FN-landende enige om 17 Verdensmål til at standse fattigdom, beskytte planeten og sikre velstand for alle. Denne persons arbejde bidrager til følgende verdensmål:
Publikation: Bidrag til tidsskrift › Tidsskriftartikel › Forskning › peer review
Publikation: Bidrag til tidsskrift › Tidsskriftartikel › Forskning › peer review
Publikation: Bidrag til tidsskrift › Review › peer review
Publikation: Bidrag til tidsskrift › Letter › peer review
Publikation: Bidrag til tidsskrift › Tidsskriftartikel › Forskning › peer review
Publikation: Bidrag til tidsskrift › Tidsskriftartikel › Forskning › peer review
Publikation: Bidrag til tidsskrift › Review › peer review
Publikation: Bidrag til tidsskrift › Tidsskriftartikel › Forskning › peer review